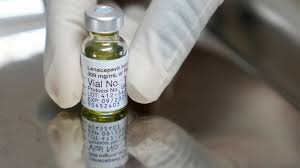
A new drug, Sunlenca (lenacapavir), has demonstrated remarkable efficacy in preventing HIV infection, offering a significant breakthrough in the fight against the virus.
Currently used as an HIV treatment, Sunlenca was tested as a pre-exposure prophylaxis (PrEP) medication in a Phase III clinical trial involving 5,000 women in South Africa and Uganda.
results show a 100% success rate in preventing HIV infection among women who received the drug every six months. Dr. Christos Petrou, Associate Professor of Pharmacology at the University of Nicosia, highlighted the significance of the findings.
The trial achieved its primary endpoint, demonstrating Sunlenca’s superiority over once-daily PrEP medications like Truvada.”
The positive outcome prompted the independent Data Monitoring Committee to recommend stopping the blinded phase of the trial and offering Sunlenca openly to all participants.
While additional research and regulatory approvals are necessary, the initial results offer hope for a highly effective tool in preventing HIV.
ALSO READ: COVID-19 cases rise among Hajj pilgrims – WHO
The study will be expanded to include men and women from diverse sexual orientations and gender identities to ensure broader applicability.
Gilead, the drug’s manufacturer, will submit applications for marketing authorization specifically for PrEP use. Regulatory approval from national and international bodies, including the World Health Organization, is crucial for making Sunlenca widely available.
If approved, Sunlenca could be a game-changer in HIV prevention strategies, potentially saving countless lives.
Since HIV was first clinically observed in 1981, over 40 million people have succumbed to the virus. This discovery offers a beacon of hope in the fight against HIV.