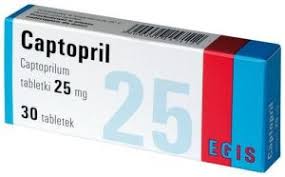
The Medicines Control Authority of Zimbabwe (MCAZ) has issued a recall for Torrent Captopril 25mg tablets, Batch No. B520K001, manufactured by Torrent Pharmaceuticals Ltd. The recall is due to the batch’s failure to meet quality standards, specifically an out-of-specification limit of Captopril disulfide.
The non-compliance may result in reduced efficacy or potential harm to patients, including:
– Reduced blood pressure control
– Increased risk of cardiovascular complications
– Adverse reactions
To ensure public health and safety, MCAZ advises:
– Licensed wholesalers and healthcare facilities: Quarantine and stop distributing the affected batch, and cooperate with the manufacturer and local distributors during the recall.
– Public members: Return the product to the pharmacy where purchased
MCAZ emphasizes the importance of using compliant medications to ensure public health and safety. The authority is working to protect the public from potential harm caused by non-compliant medications.
end//..