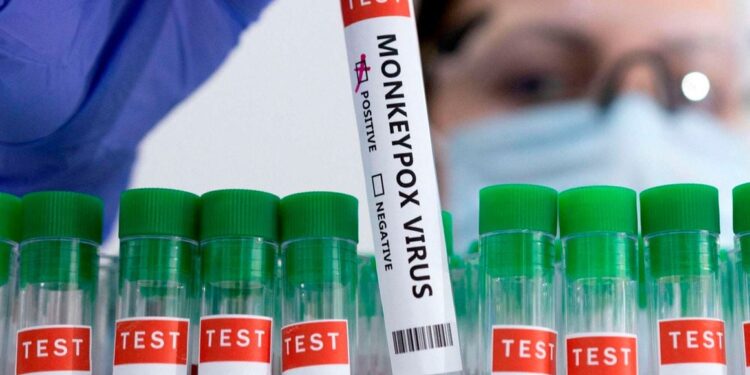
The World Health Organisation authorised Abbott Laboratories’ mpox diagnostic test for emergency use on Thursday, the first such approval in the agency’s effort to bolster testing capabilities in countries facing outbreaks of the disease.
The real-time PCR test, Alinity m MPXV assay, enables detection of mpox virus DNA from human skin lesion swabs, the WHO said, adding that it was designed for use by trained clinical laboratory personnel.
The agency said it was evaluating three new mpox diagnostic tests for emergency use and was also in discussions with other manufacturers to expand the availability of mpox diagnostic tools.
“This first mpox diagnostic test listed under the Emergency Use Listing (EUL) procedure represents a significant milestone in expanding testing availability in affected countries,” said Yukiko Nakatani, agency’s assistant director-general for access to medicines and health products.
In August, WHO asked manufacturers to submit their products for an emergency review and has been in discussion with them about the need for effective diagnostics, particularly in low-income groups.
The EUL procedure is a risk-based assessment of unlicensed vaccines, tests and treatments to expedite their availability during public health emergencies.
The WHO declared mpox a global public health emergency for the second time in two years in August, following an outbreak of the viral infection in the Democratic Republic of Congo, which has spread to neighbouring Burundi, Uganda and Rwanda.
Two strains of mpox are spreading — the clade I variant, which is endemic in parts of West and Central Africa and a new, more transmissible strain clade Ib, which has triggered global concern.
Sweden, Thailand, and India have confirmed cases of the clade Ib type of the virus outside of the Democratic Republic of Congo and neighbouring countries. Reuters