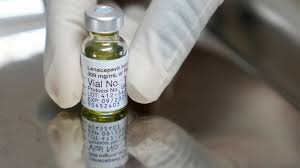
The World Health Organization (WHO) has granted its first approval for a mpox vaccine, made by Bavarian Nordic A/S, for use in adults.
This milestone enables global health organizations like Gavi and UNICEF to procure the vaccine, despite limited supplies due to a single manufacturer.
WHO Director-General Tedros Adhanom Ghebreyesus hailed the approval as a crucial step in fighting mpox in Africa, where the disease is most prevalent.
The two-dose vaccine is recommended for individuals aged 18 and above, but may be used in younger individuals in outbreak settings where benefits outweigh risks.
The vaccine has shown effectiveness in slowing the virus’ spread in adults, but its efficacy in children is less clear.
Africa has been severely affected, with over 700 deaths and 100,000 cases reported across the continent.
The Africa Center for Disease Control and Prevention emphasizes the need for approximately 10 million vaccines to combat the outbreaks, but donor countries have only pledged around 3.6 million doses so far.
Congo, the hardest-hit country, has received just 250,000 doses.
WHO is establishing an access and allocation mechanism to ensure fair distribution of mpox tests, treatments, and vaccines to those in greatest need.
The organization has launched a continental response plan with the Africa CDC to combat the ongoing outbreaks.