The World Health Organization announced on Friday that its partners, such as Gavi and UNICEF, can begin purchasing mpox vaccinations before they are certified by the United Nations health agency, allowing inoculations to reach Africa faster as the continent confronts a growing virus outbreak.
Traditionally, organisations such as Gavi, which assists low-income countries in acquiring vaccines, can only begin purchasing injections once they have received WHO approval.
However, the criteria have been modified in this case to get talks underway, as the WHO’s permission is due in a few weeks.
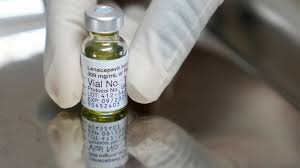
Two vaccines, developed by Denmark’s Bavarian Nordic BAVA.CO and Japan’s KM Biologics, have already been licensed by regulators worldwide, including the United States and Japan, and have been widely used to treat mpox since 2022.
Approximately 1.2 million people have received Bavarian Nordic’s vaccination in the United States alone. The WHO is scheduled to award an emergency license for the shots in September.
Mpox, a viral infection that spreads by close contact and is typically mild but fatal, was designated a public emergency of international concern by the WHO last week after a new strain of the virus spread rapidly in the Democratic Republic of Congo and elsewhere.
Earlier this month, the WHO asked vaccine makers to submit information so it could speed up the clearance process.